Terms and Agreement
Please agree to the terms to continue using the chat feature.


BioVie is a clinical-stage company developing innovative drug therapies for the treatment of neurological and neurodegenerative disorders and advanced liver disease. In neurodegenerative disease, the Company’s drug candidate bezisterim inhibits inflammatory activation that leads to neuroinflammation, and insulin resistance believed to be drivers of Alzheimer’s Disease (AD) and Parkinson’s Disease (PD). In liver disease, the Company’s Orphan drug candidate BIV201 (continuous infusion terlipressin), with U.S. FDA Fast Track status, is being evaluated and discussed with guidance received from the FDA regarding the design of Phase 3 clinical testing of BIV201 for the treatment of ascites due to chronic liver cirrhosis. The active agent is approved in the U.S. and in about 40 countries for related complications of advanced liver cirrhosis.
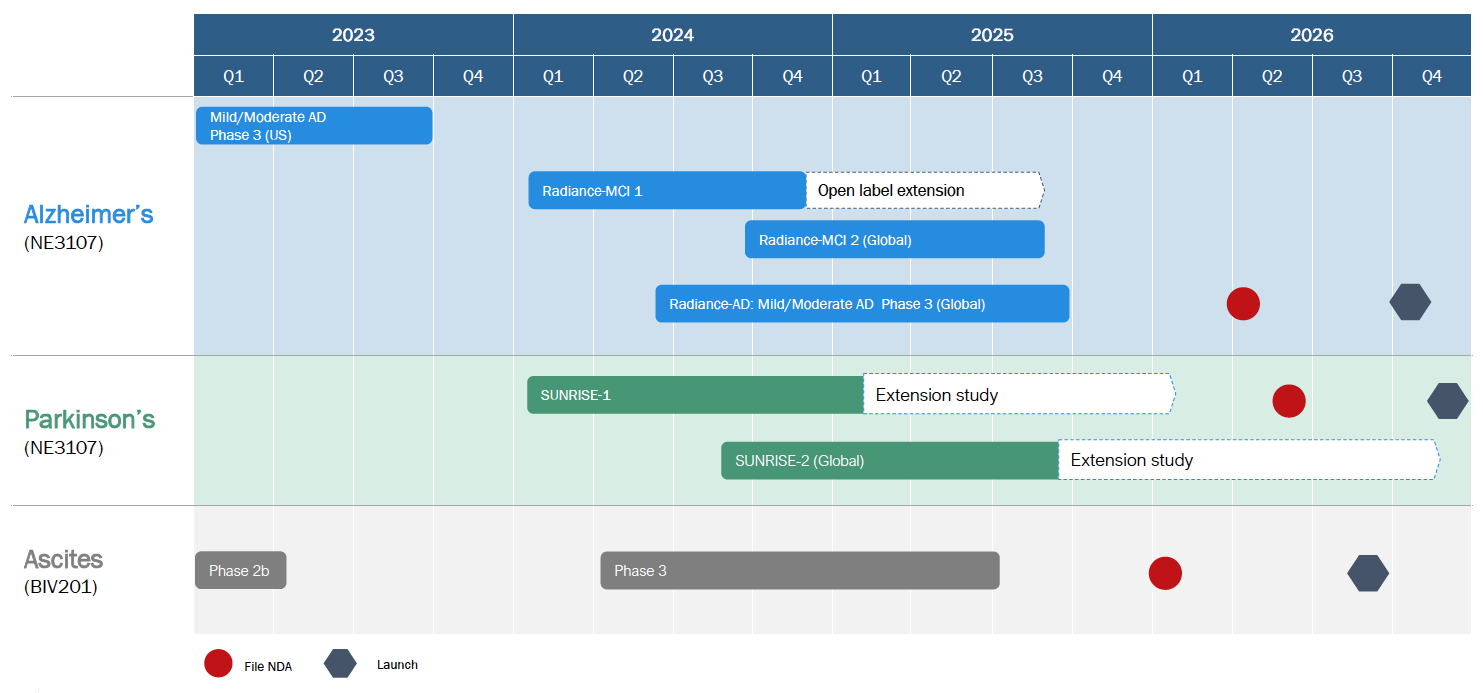
BioVie is strategically positioned for significant value creation, supported by a robust cash position and a series of anticipated catalysts over the next 12 months and beyond. Key upcoming catalysts include the launch of the Parkinson’s Phase 2b trial in Fall 2024 and the next Alzheimer’s Phase 3 trial using a new once-daily formulation of bezisterim in mid-2025. Additionally, ongoing partnership discussions for bezisterim's geographic rights and the planned launch of an ascites Phase 3 trial upon securing a partner further support the investment case for BioVie.
Lead asset bezisterim (formerly NE3107) modulates the production of TNFα. In clinical trials, many patients treated with bezisterim experienced:
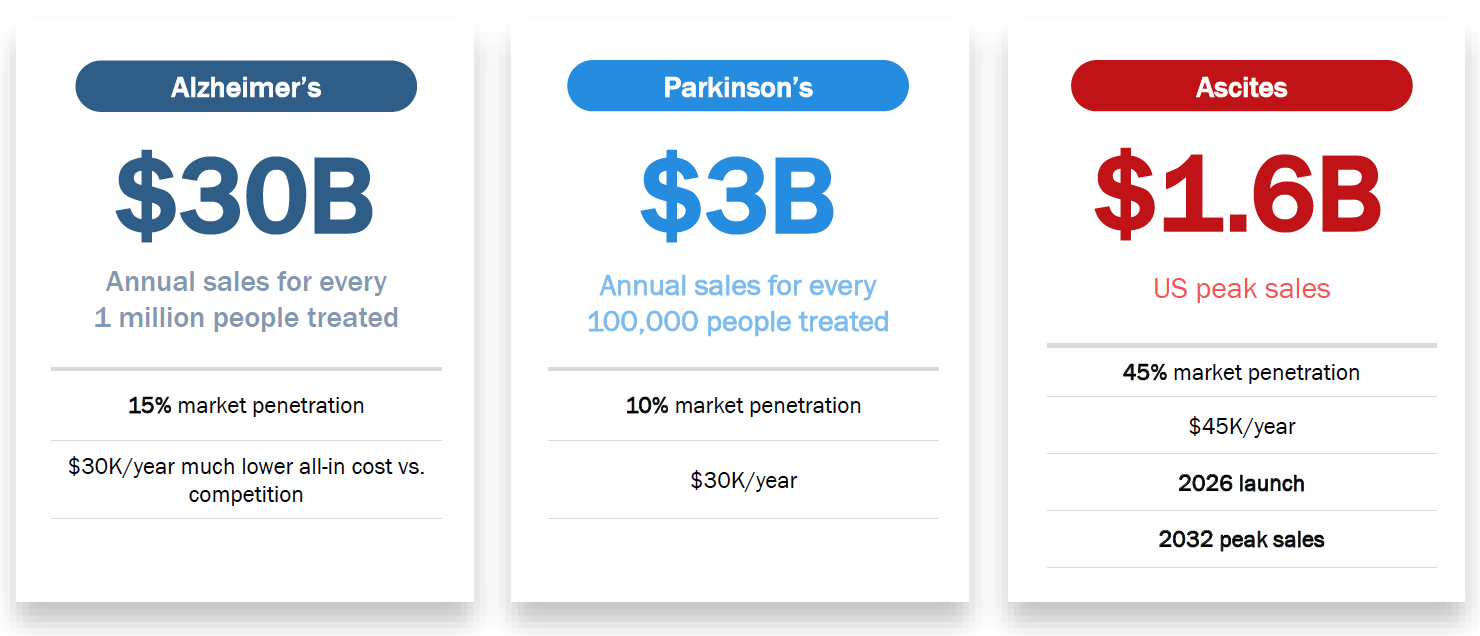
Significant unmet medical needs in PD & AD
Among competitive molecules exploring neuroinflammation and AD, bezisterim is the only one that:
BIV201 is the only drug currently in development for refractory ascites, a condition with 50% mortality rate. It has the potential to become first therapy since there are no approved drugs in the US
Upcoming catalysts
Red Chat is here to summarize the SEC filing data on BIVI
Please agree to the terms to continue using the chat feature.